Thermochromic Material
Use Thermochromic Material Technology Coloring Life
Home > Color Shifting Technologies > Thermochromic
Thermochromic Material Uses

thermochromic silicone

thermochromic fabric

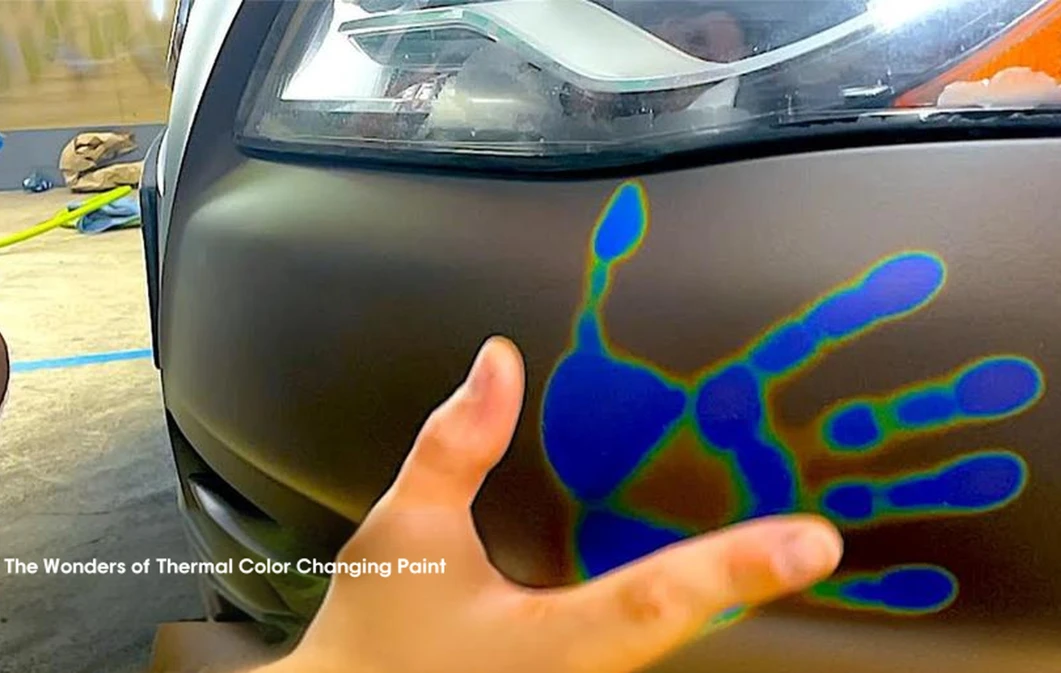
thermochromic paint on car

Thermochromic plastics

Thermochromic glass

Baby Products

Novelty Items

Craft projects

Medical

Industrial Safety

Food Packaging

Home Appliances
Thermochromic Pigment Series

KC01

KC02

KC03

KC04

KC05

KC06

KC07

KC08

KC09

KC10

kCBB-02

KCBP-13

KCBP-17

KCBP-18

KCBR-20

KCBY-04

KCCR-11

KCGY-10

KCGO-19

KCOY-01

Black

Blue

Blue-Green

Green

Purple

Orange

Pink

Red

Rose Red

Sapphire
What are the thermochromic materials?
Thermochromic materials is a kind of substance that can change color while experiencing the exterior temperature change. It displays a color change in a macroscopic aspect. When the chemical structure or physical structure changes, the color changes. The change of the substance structure can lead the change of the spectral characteristics. That is how macroscopic occurs.
1.1 Categories of Thermochromic Materials
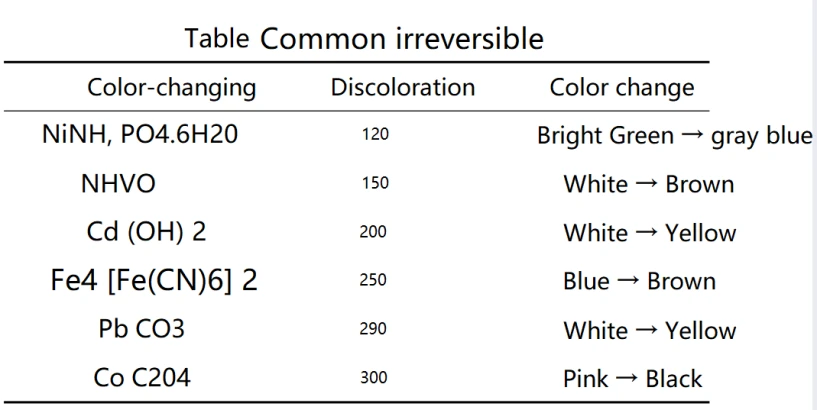
When we refer to non-reversible thermochromic materials, we generally mean a material that can experience non-reversible color change, as it can only record the highest temperature that it has experienced. When a thermochromic material is heated to a certain degree, its color will change. During this process, the color neither recovers nor changes. This kind of material is what we called thermochromic materials. There many categories of non-reversible thermochromic materials. What we commonly use are synthetic compounds such as aryl methane pigments, azo dyes, acid-based white pigments, phenol derivatives, methyl violet, as well as sulfides, oxides, nitrates, sulfates, metal phosphates (covering molybdenum, barium, magnesium, strontium, cadmium, ickel, cobalt, iron, zinc, chromium, manganese, lead).
1.2 Reversible Cholesteric Thermochromic Materials
Cholesteric liquid crystals are the main materials of reversible cholesteric thermochromic materials. Its color can change because it has spiral structure. In this structure, the interlayer spacing of the molecular layers is called spiral pitch. The spiral pitch will change once the temperature changes. Cholesteric liquid crystals with different spiral pitch will reflect light of different wavelength. That’s how cholesteric liquid crystals change colors. Cholesteric liquid crystals are main materials for liquid crystal inks to change color. Here is the specific details. Its cor-change temperature is relatively low (23~42℃) and it is sensible to color change. It is can be produced by adding binding agents after being processed by microencapsulation. By generating reaction to chemical inteference, its efficacy and sensitivity are diminished. Besides, it can realize multi-layer and continuous reversible color-changing. Regarding to its disadvantages, its production cost is rather high, so it’s expensive; the conditions for using it is rather strict, we can only use it against dark backdrop; the storage period is short due to its poor stability. These above features of the liquid crystal thermochromic materials restricts its widespread promotion and application.
1.3 Reversible Inorganic Thermochromic Materials
Reversible inorganic thermochromic material has advantages and disadvantages. First, advantages. It usually employs metallic components such as transition metal complexes, halides, oxides, metallic elements, ect. Solid inorganic thermochromic materials are suitable to be used at the temperature above 200°C. Its manufacturing cost is rather low. Other advantages include sound manufacturing performance, robust thermal and photostability. Then, we come to its disadvantages. Its color and temperature for color-changing are hardly to be controlled and plus, its color-changing ability is restricted by its intrinsic properties. Besides, it is highly corrosive and toxic. Therefore, its application is not extensive.
1.4 Reversible Organic Thermochromic Materials
There are plenty of materials that belongs to reversible organic thermochromic materials. According to the name of organic compounds, they can classified into α-naphthoquinone derivatives, bisanthrones, spirooxindoles, fulgides, spiropyrans, indolenine phthaleins, triarylmethane phthaleins, and ect. According to their elements, they can be classified into two categories. The fist category is multi-component compounded thermochromic materials. Its advantage are as follows. Its thermochromic range is 20~200℃. This kind of material is emerging as a new material. Its manufacturing cost is low. It is obvious when the color changes, with vivid color features and high sensitivity to color change. The second category is single-component thermochromic materials, which is featured by a single substance.
Thermochromic Mechanisms of Thermochromic Materials
2.1 Non-Reversible Thermochromic Materials
The operational temperature for non-reversible thermochromic materials is about 30 ~1200℃. When the temperature rises, non-reversible thermochromic materials will display chemical changes and non-reversible physical changes. The mechanisms for thermochromism of this kind of materials are as follows.
- Solid-state reactions. During this reaction, the color of reactors is totally different from that of their resulting products. This reaction happens when under the same temperature, two or more than two mixed compounds enter into solid-state reaction.
- Thermal decomposition When heated, the substance enters into thermal decompositionreaction. The color of the substance changes duo to the difference of its chemical structure before and after the decomposition.
- Oxidative transformations. Under the oxygen circumstance, when heated, some substances enter into oxidation reactions and generate new oxides. There is change of color during this process.
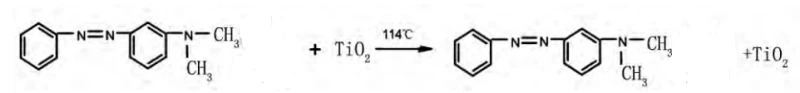
- Melting-induced color change. Under certain circumstances, the organic crystalline materialsenters into melting-induced The structure of the organic crystalline materials is damaged. The crystal particles are active and move in an irregular level. The organic crystalline materials transforms from non-transparent solid-state to transparent melting state. There are apparent color changes before and after the melting. For example, there are tita.nium dioxide and dimethylaminoazobenzene.
2.2 Color Change Mechanism in Reversible Thermochromic Liquid Crystal Materials
Thermochromic liquid crystal materials can selectively reflect the polarized light of certain wave bands and absorb the light of certain wave bands. The color and wavelength of the the reflection light and transmitted light on the surface of the liquid crystal will change when the spiral structure subtracts or extends. The spiral structure is sensitive to the temperature and its subtracting or extending are greatly influenced by the exterior temperature. Hence, thermochromic liquid crystal materials can, in a certain temperature range, reversibly exhibit color in a entire range of visible light along with the temperature change.
2.3 Color Change Mechanism in Reversible Thermochromic Liquid Crystal Materials
2.3.1 Transformation of Crystalline Structure
Reversible inorganic thermochromic materials will, under certain temperature, experience color changes. It will also enter into the crystalline structure transformation. When the temperature cools down, the color will revert into its original state and the crystalline structure will also revert into its initial state. For most metal ion compounds, their color changes are induced by transformation of crystalline structure. For instance;
2.3.2 Loss and Reabsorption of Crystalline Water
When heated to a certain temperature, reversible inorganic thermochromic materials with crystalline water will loss crystalline water and the color will change. When the temperature cools down, the reversible inorganic thermochromic materials will re-start to absorb water from exterior environment, the color will revert back to its original state. For instance:
2.3.3 Electron Transfer
Some reversible inorganic thermochromic materials will experience oxidation-reduction reaction under certain temperature. This kind of reaction will enable the electrons to transfer among different elements, thus generate new substance. During this process, the color will change. Subsequently, when the exterior environment impact disappears, the new substance also disappears. The color will revert to its original state. For example, The temperature for PbCrO4 chromotropic coating to change color is around 1000°C. During the color-changing process, there is a distinct change of color; the color is reversible with rather high accuracy.
2.3.4 Changes in Ligand Geometry
Inorganic reversible organic thermochromic materials has pronounced differences in its color. But its heat resistance property is sound. This kind of material is stable in its attributes. When the exterior temperature changes, the ligand geometry of this material will undergo reversible changes, thus leading to the reversible change of the color. For instance:
2.4 The Color Variation Mechanism of Organic Reversible Thermochromic Materials
2.4.1 Electron Transfer Mechanism
When the exterior temperature changes, there will be electron transfer inside the substance. The substance absorb or radiate a certain wavelength of light, thus leading the reversible change of the substance color. This kind of thermochromic materials which possess such color changing mechanism are composed of solvent-mimicking entities, electron acceptors, electron donors. Take the color change of Bisphenol A and Crystal Violet Lactone as an example.
When the exterior temperature changes, the structural reorganization of this kind of thermochromic materials will undergo reversible change, thus leading the reversible change of the substance. For example, when heated, the structural reorganization of the solid complex Ni(N, N’-dimethylvinyldiamine) 2(NO2)] (H2O) will change. The color will change from red to blue as illustrated in Figure below.
[Ni( N,N’- dimethylvinyldiamine) 2 (NO2) ]
2.4.3 Tautomeric Interconversion
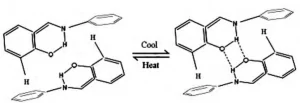
The color-changing of this kind of reversible thermochromic materials can attribute to the tautomeric interconversion of the ketone form and enol form. This kind of material is mainly synthesized by phenanthrene aldehyde, naphthaldehyde, ortho-hydroxy derivatives of benzaldehyde, and their respective analogs. The tautomeric interconversion of Salicylidene aniline are respectively ketonic forms and enolic forms. There is a balance of temperature sensitivity between the two forms, as shown in Figure below. Salicylidene aniline is a kind of a compound with a Schiff base nature and a catecholic backbone. When the temperature rises, the enolic structure enriches; conversely, when the temperature drops, the ketonic structure decreases. The change of temperature leads to the change of color.
The mechanism of reversible thermochromism in salicylidene aniline
2.4.4 Thermal Motion of Molecular Chains
Recent years, people have studied the reversible thermochromic materials by polydiacetylene derivatives assembled by covalent bonds or aromatic interactions, and by enhanced hydrogen bonding. Many thermochromisms polydiacetylene derivative are irreversible. Layered, reversible thermochromic polydiyne derivatives assembled via hydrogen bonding self-organization alter their hue in response to variations in the ambient thermal conditions. When the exterior temperature changes, the thermal motion of molecular chain will trigger the color change. For polydiacetylene derivatives made by using the method of photopolymerization, when the temperature rises, conjugated system’s length will contract. This process lead the absorption spectrum transfer from blue to orange. When the temperature cools, the color reverts to be blue.
Scientific researchers have already grasped deep knowledge about thermochromic materials after hundred years of exploration and development. We have developed many thermochromic materials including Polymeric, liquid crystalline, organic, inorganic varieties. The products of this series have been extensively applied in people’s daily life and industrial sectors.
Table of Contents
- What are the thermochromic materials?
- 1.1 Categories of Thermochromic Materials
- 1.2 Reversible Cholesteric Thermochromic Materials
- 1.3 Reversible Inorganic Thermochromic Materials
- 1.4 Reversible Organic Thermochromic Materials
- Thermochromic Mechanisms of Thermochromic Materials
- 2.1 Non-Reversible Thermochromic Materials
- 2.2 Color Change Mechanism in Reversible Thermochromic Liquid Crystal Materials
- 2.3 Color Change Mechanism in Reversible Thermochromic Liquid Crystal Materials
- 2.4 The Color Variation Mechanism of Organic Reversible Thermochromic Materials
We are Ready to Support Your Thermochromic Material Projects
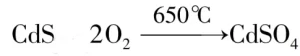

![[Ni( N,N’- dimethylvinyldiamine) 2 (NO2) ]](https://www.kingchroma.com/wp-content/uploads/2024/09/图片11-300x172.webp)